Systemic lupus erythematosus (SLE or lupus, pronounced /s??st?m?k ?lu?p?s ??r???im??to?s?s/) is a chronic autoimmune disease that can be fatal, though with recent medical advances, fatalities are becoming increasingly rare. As with other autoimmune diseases, the immune system attacks the body’s cells and tissue, resulting in inflammation and tissue damage. SLE can affect any part of the body, but most often harms the heart, joints, skin, lungs, blood vessels, liver, kidneys, and nervous system. The course of the disease is unpredictable, with periods of illness (called flares) alternating with remissions. Lupus can occur at any age, and is most common in women, particularly of non-European descent.[1] Lupus is treatable symptomatically, mainly with corticosteroids and immunosuppressants, though there is currently no cure. Survival in patients with SLE in the United States, Canada, and Europe is approximately 95% at 5 years, 90% at 10 years, and 78% at 20 years.[2]
Classification of Systemic lupus erythematosus
Lupus is a chronic autoimmune disease. Clinically, it can affect multiple organ systems, including the heart, skin, joints, kidneys, and nervous system. There are several types of lupus; generally, when the word “lupus” alone is used, it refers to systemic lupus erythematosus or SLE, as discussed in this article. Other types include:
- Drug-induced lupus erythematosus, a drug-induced form of SLE; this type of lupus can occur equally in either sex.
- Lupus nephritis, an inflammation of the kidneys caused by SLE.
- Discoid lupus erythematosus, a skin disorder that causes a red, raised rash on the face and scalp. Discoid lupus occasionally (1–5%) develops into SLE.[3]
- Subacute cutaneous lupus erythematosus, which causes nonscarring skin lesions on patches of skin exposed to sunlight.[4]
- Neonatal lupus, a rare disease affecting babies born to women with SLE, Sjögren’s syndrome, or sometimes no autoimmune disorder. It is theorized that maternal antibodies attack the fetus, causing skin rash; liver problems; low blood counts, which gradually fade; and heart block, leading to bradycardia.[4]
Signs and symptoms of Systemic lupus erythematosus
SLE is one of several diseases known as “the great imitators”[5] because its symptoms vary so widely, it often mimics or is mistaken for other illnesses and because the symptoms come and go unpredictably. Diagnosis can be elusive, with patients sometimes suffering unexplained symptoms and untreated SLE for years. Common initial and chronic complaints are fever, malaise, joint pains, myalgias, fatigue and temporary loss of cognitive abilities. Because they are so often seen with other diseases, these signs and symptoms are not part of the diagnostic criteria for SLE. When occurring in conjunction with other signs and symptoms (see below), however, they are considered suggestive.[6]
Common symptoms explained
- Dermatological manifestations
- As many as 30% of patients present with some dermatological symptoms (and 65% suffer such symptoms at some point), with 30% to 50% suffering from the classic malar rash (or butterfly rash) associated with the disease. Patients may present with discoid lupus (thick, red scaly patches on the skin). Alopecia; mouth, nasal, and vaginal ulcers; and lesions on the skin are also possible manifestations.
- Musculoskeletal manifestations
- Patients most often seek medical attention for joint pain, with the small joints of the hand and wrist usually affected, although all joints are at risk. The Lupus Foundation of America estimates that more than 90 percent will experience joint and/or muscle pain at some time during the course of their illness.[7] Unlike rheumatoid arthritis, lupus arthritis is less disabling and usually does not cause severe destruction of the joints. Fewer than ten percent of people with lupus arthritis will develop deformities of the hands and feet.[7]
- Hematological manifestations
- Anemia and iron deficiency may develop in as many as half of patients. Low platelet and white blood cell counts may be due to the disease or a side effect of pharmacological treatment. Patients may have an association with antiphospholipid antibody syndrome (a thrombotic disorder) wherein autoantibodies to phospholipids are present in the patient’s serum. Abnormalities associated with antiphospholipid antibody syndrome include a paradoxical prolonged PTT (which usually occurs in hemorrhagic disorders) and a positive test for antiphospholipid antibodies; the combination of such findings have earned the term “lupus anticoagulant positive.” Another autoantibody finding in lupus is the anticardiolipin antibody, which can cause a false positive test for syphilis.
- Cardiac manifestations
- Patients may present with inflammation of various parts of the heart, such as pericarditis, myocarditis, and endocarditis. The endocarditis of SLE is characteristically noninfective (Libman-Sacks endocarditis) and involves either the mitral valve or the tricuspid valve. Atherosclerosis also tends to occur more often and advances more rapidly in SLE patients than in the general population.[8][9][10]
- Pulmonary manifestations
- Lung and pleura inflammation can cause pleuritis, pleural effusion, lupus pneumonitis, chronic diffuse interstitial lung disease, pulmonary hypertension, pulmonary emboli, pulmonary hemorrhage and shrinking lung syndrome.
- Hepatic involvement
- See autoimmune hepatitis.
- Renal involvement
- Painless hematuria or proteinuria may often be the only presenting renal symptom. Acute or chronic renal impairment may develop with lupus nephritis, leading to acute or end stage renal failure. Because of early recognition and management of SLE, end stage renal failure occurs in less than 5% of patients.
- Histologically, a hallmark of SLE is membranous glomerulonephritis with “wire loop” abnormalities.[11] This finding is due to immune complex deposition along the glomerular basement membrane, leading to a typical granular appearance in immunofluorescence testing.
- Neurological manifestations
- About 10% of patients may present with seizures or psychosis. One-third may test positive for abnormalities in the cerebrospinal fluid.
- T-cell abnormalities
- Abnormalities in T cell-signaling are associated with SLE, including a deficiency in CD45 phosphatase and increased expression of CD40 ligand.
- Other rarer manifestations
- Lupus gastroenteritis, lupus pancreatitis, lupus cystitis, autoimmune inner ear disease, parasympathetic dysfunction, retinal vasculitis, and systemic vasculitis.
Other abnormalities include:
- Increased expression of Fc?RI?, which replaces the sometimes deficient TCR ? chain
- Increased and sustained calcium levels in T cells
- Moderate increase of inositol triphosphate
- Reduction in PKC phosphorylation
- Reduction in Ras-MAP kinase signaling
- Deficiencies in protein kinase A I activity
Causes of Systemic lupus erythematosus
- Transmission
In SLE, the body’s immune system produces antibodies against itself, particularly against proteins in the cell nucleus. SLE is triggered by environmental factors that are unknown (but probably include viruses) in people with certain combinations of genes in their immune system.
“All the key components of the immune system are involved in the underlying mechanisms” of SLE, according to Rahman, and SLE is the prototypical autoimmune disease. The immune system must have a balance (homeostasis) between being sensitive enough to protect against infection, and being too sensitive and attacking the body’s own proteins (autoimmunity). From an evolutionary perspective, according to Crow, the population must have enough genetic diversity to protect itself against a wide range of possible infection; some genetic combinations result in autoimmunity. The likely environmental triggers include ultraviolet light, drugs, and viruses. These stimuli cause the destruction of cells and expose their DNA, histones, and other proteins, particularly parts of the cell nucleus. Because of genetic variations in different components of the immune system, in some people the immune system attacks these nuclear-related proteins and produces antibodies against them. Ultimately, these antibody complexes damage blood vessels in critical areas of the body, such as the glomeruli of the kidney; these antibody attacks are the cause of SLE. Researchers are now identifying the individual genes, the proteins they produce, and their role in the immune system. Each protein is a link on the autoimmune chain, and researchers are trying to find drugs to break each of those links. [12][13][14]
SLE is a chronic inflammatory disease believed to be a type III hypersensitivity response with potential type II involvement.[15]
- Genetics
- The first mechanism may arise genetically. Research indicates that SLE may have a genetic link. Lupus does run in families, but no single “lupus gene” has yet been identified. Instead, multiple genes appear to influence a person’s chance of developing lupus when triggered by environmental factors. The most important genes are located on chromosome 6, where mutations may occur randomly (de novo) or may be inherited. Additionally, people with SLE have an altered RUNX-1 binding site, which may be either cause or contributor (or both) to the condition. Altered binding sites for RUNX-1 have also been found in people with psoriasis and rheumatoid arthritis.
- Environmental triggers
- The second mechanism may be due to environmental factors. These factors may not only exacerbate existing lupus conditions but also trigger the initial onset. They include certain medications (such as some antidepressants and antibiotics), extreme stress, exposure to sunlight, hormones, and infections. Some researchers have sought to find a connection between certain infectious agents (viruses and bacteria), but no pathogen can be consistently linked to the disease. UV radiation has been shown to trigger the photosensitive lupus rash, but some evidence also suggests that UV light is capable of altering the structure of the DNA, leading to the creation of autoantibodies. Some researchers have found that women with silicone gel-filled breast implants have produced antibodies to their own collagen, but it is not known how often these antibodies occur in the general population, and there is no data that show that these antibodies cause connective tissue diseases such as lupus.
- Drug reactions
- Drug-induced lupus erythematosus is a reversible condition that usually occurs in patients being treated for a long-term illness. Drug-induced lupus mimics systemic lupus. However, symptoms of drug-induced lupus generally disappear once a patient is taken off the medication that triggered the episode. There are about 400 medications currently in use that can cause this condition, though the most common drugs are procainamide, hydralazine, quinidine and Phenytoin.
- Non-SLE forms of lupus
- Discoid (cutaneous) lupus is limited to skin symptoms and is diagnosed by biopsy of skin rash on the face, neck, or scalp. Often an antinuclear antibody (ANA) test for discoid patients is negative or a low-titer positive. About 1–5% of discoid lupus patients eventually develop SLE.
- Clearance deficiency
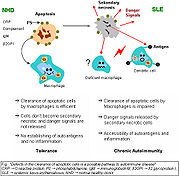
The exact mechanisms for the development of systemic lupus erythematosus (SLE) are still unclear, since the pathogenesis is a multifactorial event. Beside discussed causations, impaired clearance of dying cells is a potential pathway for the development of this systemic autoimmune disease. This includes deficient phagocytic activity and scant serum components in addition to increased apoptosis.
Monocytes isolated from whole blood of SLE patients show reduced expression of CD44 surface molecules involved in the uptake of apoptotic cells. Most of the monocytes and tingible body macrophages (TBM), which are found in the germinal centres of lymph nodes, even show a definitely different morphology in patients with SLE; they are smaller or scarce and die earlier. Serum components like complement factors, CRP, and some glycoproteins are furthermore decisively important for an efficiently operating phagocytosis. In patients, these components are often missing, diminished, or inefficient.
The clearance of early apoptotic cells is an important function in multicellular organisms. It leads to a progression of the apoptosis process and finally to secondary necrosis of the cells if this ability is disturbed. Necrotic cells release nuclear fragments as potential autoantigens as well as internal danger signals, inducing maturation of dendritic cells (DC), since they have lost their membranes’ integrity. Increased appearance of apoptotic cells also simulates inefficient clearance. That leads to maturation of DC and also to the presentation of intracellular antigens of late apoptotic or secondary necrotic cells, via MHC molecules. Autoimmunity possibly results by the extended exposure to nuclear and intracellular autoantigens derived from late apoptotic and secondary necrotic cells. B and T cell tolerance for apoptotic cells is abrogated, and the lymphocytes get activated by these autoantigens; inflammation and the production of autoantibodies by plasma cells is initiated. A clearance deficiency in the skin for apoptotic cells has also been observed in patients with cutaneous lupus erythematosus (CLE).[16]
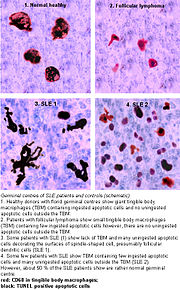
Germinal centres.
Accumulation in germinal centres (GC)
In healthy conditions, apoptotic lymphocytes are removed in germinal centres by specialised phagocytes, the tingible body macrophages (TBM); that’s why no free apoptotic and potential autoantigenic material can be seen. In some patients with SLE, accumulation of apoptotic debris can be observed in GC because of an ineffective clearance of apoptotic cells. In close proximity to TBM, follicular dendritic cells (FDC) are localised in GC, which attach antigen material to their surface and, in contrast to bone marrow-derived DC, neither take it up nor present it via MHC molecules. Autoreactive B cells can accidentally emerge during somatic hypermutation and migrate into the GC light zone. Autoreactive B cells, maturated coincidentally, normally don’t receive survival signals by antigen planted on follicular dendritic cells and perish by apoptosis. In the case of clearance deficiency, apoptotic nuclear debris accumulates in the light zone of GC and gets attached to FDC. This serves as a germinal centre survival signal for autoreactive B-cells. After migration into the mantle zone, autoreactive B cells require further survival signals from autoreactive helper T cells, which promote the maturation of autoantibody-producing plasma cells and B memory cells. In the presence of autoreactive T cells, a chronic autoimmune disease may be the consequence.
Pathophysiology
One manifestation of lupus is abnormalities in apoptosis, a type of programmed cell death in which aging or damaged cells are neatly disposed of as a part of normal growth or functioning.
Abnormalities in apoptosis
- Apoptosis is increased in monocytes and keratinocytes;
- Expression of Fas by B cells and T cells is increased;
- There are correlations between the apoptotic rates of lymphocytes and disease activity.
Tingible body macrophages (TBMs) are large phagocytic cells in the germinal centers of secondary lymph nodes; they express CD68 protein. These cells normally engulf B cells that have undergone apoptosis after somatic hypermutation. In some patients with SLE, significantly fewer TBMs can be found, and these cells rarely contain material from apoptotic B cells. Also, uningested apoptotic nuclei can be found outside of TBMs. This material may present a threat to the tolerization of B cells and T cells. Dendritic cells in the germinal center may endocytose such antigenic material and present it to T cells, activating them. Also, apoptotic chromatin and nuclei may attach to the surfaces of follicular dendritic cells and make this material available for activating other B cells that may have randomly acquired self-specificity through somatic hypermutation.[17]
Diagnosis for Systemic lupus erythematosus
Microphotograph of a histological section of human skin prepared for direct immunofluorescence using an anti-IgG antibody. The skin is from a patient with systemic lupus erythematosus and shows IgG deposits at two different places: the first is a bandlike deposit along the epidermal basement membrane (“lupus band test” is positive); the second is within the nuclei of the epidermal cells (antinuclear antibodies are present).
Some physicians make a diagnosis on the basis of the ACR classification criteria (see below). The criteria, however, were established mainly for use in scientific research (i.e., inclusion in randomized controlled trials), and patients may have lupus but never meet the full criteria.
Antinuclear antibody testing and anti-extractable nuclear antigen (anti-ENA) form the mainstay of serologic testing for lupus. Antiphospholipid antibodies occur more often in SLE and can predispose for thrombosis. More specific are the anti-Smith and anti-dsDNA antibodies. Other tests routinely performed in suspected SLE are complement system levels (low levels suggest consumption by the immune system), electrolytes and renal function (disturbed if the kidney is involved), liver enzymes, and a complete blood count.
Previously, the lupus erythematosus (LE) cell test was not commonly used for diagnosis because those LE cells are only found in 50–75% of SLE patients, and are also found in some patients with rheumatoid arthritis, scleroderma, and drug sensitivities. Because of this, the LE cell test is now performed only rarely and is mostly of historical significance.[18]
Diagnostic criteria
The American College of Rheumatology (ACR) established eleven criteria in 1982,[19] which were revised in 1997[20] as a classificatory instrument to operationalise the definition of SLE in clinical trials. They were not intended to be used to diagnose individual patients and do not do well in that capacity. For inclusion in clinical trials, patients must meet the following three criteria to be classified as having SLE: (i) patient must present with four of the below eleven symptoms, (ii) either simultaneously or serially, (iii) during a given period of observation.
- Serositis: Pleuritis (inflammation of the membrane around the lungs) or pericarditis (inflammation of the membrane around the heart); sensitivity = 56%; specificity = 86% (pleural is more sensitive; cardiac is more specific).[21]
- Oral ulcers (includes oral or nasopharyngeal ulcers).
- Arthritis: nonerosive arthritis of two or more peripheral joints, with tenderness, swelling, or effusion; sensitivity = 86%; specificity = 37%.[21]
- Photosensitivity (exposure to ultraviolet light causes skin rash, or other symptoms of Lupus flareups); sensitivity = 43%; specificity = 96%.[21]
- Blood—hematologic disorder—hemolytic anemia (low red blood cell count) or leukopenia (white blood cell count<4000/µl), lymphopenia (<1500/µl) or thrombocytopenia (<100000/µl) in the absence of offending drug; sensitivity = 59%; specificity = 89%.[21] Hypocomplementemia is also seen, due to either consumption of C3 and C4 by immune complex-induced inflammation or to congenitally complement deficiency, which may predispose to SLE.
- Renal disorder: More than 0.5g per day protein in urine or cellular casts seen in urine under a microscope; sensitivity = 51%; specificity = 94%.[21]
- Antinuclear antibody test positive; sensitivity = 99%; specificity = 49%.[21]
- Immunologic disorder: Positive anti-Smith, anti-ds DNA, antiphospholipid antibody, and/or false positive serological test for syphilis; sensitivity = 85%; specificity = 93%.[21] Presence of anti-ss DNA in 70% of patients (though also positive in patients with rheumatic disease and healthy persons[22]).
- Neurologic disorder: Seizures or psychosis; sensitivity = 20%; specificity = 98%.[21]
- Malar rash (rash on cheeks); sensitivity = 57%; specificity = 96%.[21]
- Discoid rash (red, scaly patches on skin that cause scarring); sensitivity = 18%; specificity = 99%.[21]
A useful mnemonic for these 11 criteria is SOAP BRAIN MD: Serositis (8), Oral ulcers (4), Arthritis (5), Photosensitivity (3), Blood Changes (9), Renal involvement (proteinuria or casts) (6), ANA (10), Immunological changes (11), Neurological signs (seizures, frank psychosis) (7), Malar Rash (1), Discoid Rash (2).
Some patients, especially those with antiphospholipid syndrome, may have SLE without four criteria, and SLE is associated with manifestations other than those listed in the criteria.[23][24][25]
Alternative criteria
Recursive partitioning has been used to identify more parsimonious criteria.[21] This analysis presented two diagnostic classification trees:
1. Simplest classification tree: LSE is diagnosed if the patient has an immunologic disorder (anti-DNA antibody, anti-Smith antibody, false positive syphilis test, or LE cells) or malar rash.
- sensitivity = 92%
- specificity = 92%
2. Full classification tree: Uses 6 criteria.
- sensitivity = 97%
- specificity = 95%
Other alternative criteria have been suggested.[26]
Treatment of Systemic lupus erythematosus
As lupus erythematosus is a chronic disease with no known cure, treatment is restricted to dealing with the symptoms. Essentially, this involves preventing flares and reducing their severity and duration when they occur. There are several means of preventing and dealing with flares, including drugs, alternative medicine, and lifestyle changes.
Drug therapy
Due to the variety of symptoms and organ system involvement with lupus patients, the severity of the SLE in a particular patient must be assessed in order to successfully treat SLE. Mild or remittent disease can sometimes be safely left untreated. If required, nonsteroidal anti-inflammatory drugs and antimalarials may be used.
Disease-modifying antirheumatic drugs (DMARDs) are used preventively to reduce the incidence of flares, the process of the disease, and lower the need for steroid use; when flares occur, they are treated with corticosteroids. DMARDs commonly in use are antimalarials and immunosuppressants (e.g., methotrexate and azathioprine). Hydroxychloroquine (trade name Plaquenil) is an FDA-approved antimalarial used for constitutional, cutaneous, and articular manifestations, while cyclophosphamide (trade names Cytoxan and Neosar) is used for severe glomerulonephritis or other organ-damaging complications. In 2005, mycophenolic acid (trade name CellCept) became accepted for treatment of lupus nephritis.
In more severe cases, medications that modulate the immune system (primarily corticosteroids and immunosuppressants) are used to control the disease and prevent recurrence of symptoms (known as flares). Patients who require steroids frequently may develop obesity, diabetes mellitus, diabetes, and osteoporosis. Depending on the dosage, corticosteroids can cause other side effects, such as a puffy face, an unusually large appetite, and difficulty sleeping. Those side effects can subside if and when the large initial dosage is reduced, but long-term use of even low doses can cause elevated blood pressure and cataracts. Due to these side effects, steroids are avoided if possible.
Since a large percentage of lupus patients suffer from varying amounts of chronic pain, stronger prescription analgesics may be used if over-the-counter drugs (mainly nonsteroidal anti-inflammatory drugs) do not provide effective relief. Moderate pain in lupus patients is typically treated with mild prescription opiates such as dextropropoxyphene (trade name Darvocet) and co-codamol (trade name Tylenol #3). Moderate to severe chronic pain is treated with stronger opioids, such as hydrocodone (trade names Lorcet, Lortab, Norco, Vicodin, Vicoprofen) or longer-acting continuous-release opioids, such as oxycodone (trade name OxyContin), MS Contin, or Methadone. The Fentanyl Duragesic Transdermal patch is also a widely used treatment option for the chronic pain of lupus complications because of its long-acting timed release and ease of use. When opioids are used for prolonged periods, drug tolerance, chemical dependency, and (rarely) addiction may occur. Opiate addiction is not typically a concern for lupus patients, since the condition is not likely to ever completely disappear. Thus, lifelong treatment with opioids is fairly common in lupus patients who exhibit chronic pain symptoms, accompanied by periodic titration that is typical of any long-term opioid regimen.
Lifestyle changes
Other measures, such as avoiding direct sunlight, covering up with sun-protective clothing, and using strong UVA/UVB sunblock lotion can also be effective in preventing photosensitivity problems. Weight loss is also recommended in overweight and obese patients to alleviate some of the effects of the disease, especially where joint involvement is significant.
Prevention for Systemic lupus erythematosus
Lupus is not understood well enough to be prevented, but when the disease develops, quality of life can be improved through flare prevention. The warning signs of an impending flare include increased fatigue, pain, rash, fever, abdominal discomfort, headache, and dizziness. Early recognition of warning signs and good communication with a doctor can help individuals with lupus remain active, experience less pain, and reduce medical visits.[4]
Complications during pregnancy
While most infants born to mothers with lupus are healthy, pregnant mothers with SLE should remain under a doctor’s care until delivery. Neonatal lupus is rare, but identification of mothers at highest risk for complications allows for prompt treatment before or after birth. In addition, SLE can flare during pregnancy, and proper treatment can maintain the health of the mother longer. Women pregnant and known to have the antibodies for anti-Ro (SSA) or anti-La (SSB) should have echocardiograms during the 16th and 30th weeks of pregnancy to monitor the health of the heart and surrounding vasculature.[4]
Prognosis
In the 1950s, most patients diagnosed with SLE lived fewer than five years. Advances in diagnosis and treatment have improved survival to the point where over 90% of patients now survive for more than ten years, and many can live relatively asymptomatically. The most common cause of death is infection due to immunosuppression as a result of medications used to manage the disease. Prognosis is normally worse for men and children than for women; fortunately, if symptoms are present after age 60, the disease tends to run a more benign course. The ANA is the most sensitive screening test, while anti-Sm (anti-Smith) is the most specific. The ds-DNA (double-stranded DNA) antibody is also fairly specific and often fluctuates with disease activity; the ds-DNA titer is therefore sometimes useful to diagnose or monitor acute flares or response to treatment.[27]
Epidemiology
Previously believed to be a rare disease, lupus has seen an increase in awareness and education since the 1960s. This has helped many more patients get an accurate diagnosis, making it possible to estimate the number of people with lupus with some certainty. In the United States alone, it is estimated that between 270,000 and 1.5 million people have lupus, making it more common than cystic fibrosis or cerebral palsy. The disease affects both females and males, though young women are diagnosed nine times more often than men. SLE occurs with much greater severity among African-American women, who suffer more severe symptoms as well as a higher mortality rate.[28] Worldwide, a conservative estimate states that over 5 million people have lupus.
Although SLE can occur in anyone, at any age, it is most common in women of childbearing age. It affects 1 in 4000 people in the United States, again with women becoming afflicted far more often than men. The disease appears to be more prevalent in women of African, Asian, Hispanic, and Native American origin, but this may be due to socioeconomic factors. People with relatives who suffer from SLE, rheumatoid arthritis, or thrombotic thrombocytopenic purpura are at a slightly higher risk than the general population.
History
Medical historians have theorized that people with porphyria (a disease that shares many symptoms with lupus) generated folklore stories of vampires and werewolves, due to the photosensitivity, scarring, hair growth, and porphyrin brownish-red stained teeth in severe recessive forms of porphyria (or combinations of the disorder, known as dual, homozygous, or compound heterozygous porphyrias).
The history of lupus erythematosus can be divided into three periods: classical, neoclassical, and modern. The classical period began when the disease was first recognized in the Middle Ages and saw the description of the dermatological manifestation of the disorder. The term lupus is attributed to 12th-century physician Rogerius, who used it to describe the classic malar rash. The neoclassical period was heralded by Móric Kaposi‘s recognition in 1872 of the systemic manifestations of the disease. The modern period began in 1948 with the discovery of the LE cell (the lupus erythematosus cell—a misnomer, as it occurs with other diseases as well) and is characterised by advances in our knowledge of the pathophysiology and clinical-laboratory features of the disease, as well as advances in treatment.
Useful medication for the disease was first found in 1894, when quinine was first reported as an effective therapy. Four years later, the use of salicylates in conjunction with quinine was noted to be of still greater benefit. This was the best available treatment to patients until the middle of the twentieth century, when Hench discovered the efficacy of corticosteroids in the treatment of SLE.
In modern times, the disease has received a degree of notoriety due to it becoming the subject of a running joke on the popular television series House, M.D.. In each episode when a patient with an undiagnosable condition is presented, Lupus is routinely suggested as a possible early diagnosis, despite the fact that it seldom is the answer.
Origins of “lupus erythematosus”
There are several explanations ventured for the term lupus erythematosus. Lupus is Latin for wolf, and “erythro” is derived from ???????, Greek for “red.” All explanations originate with the reddish, butterfly-shaped malar rash that the disease classically exhibits across the nose and cheeks.
- In various accounts, some doctors thought the rash resembled the pattern of fur on a wolf’s face.
- In other accounts, doctors thought that the rash, which was often more severe in earlier centuries, created lesions that resembled wolf bites or scratches.
- Another account claims that the term “lupus” did not come from Latin at all, but from the term for a French style of mask that women reportedly wore to conceal the rash on their faces. The mask is called a “loup,” French for “wolf.”
- Another common explanation for the term is that the disease’s course involves repeated attacks like those of a voracious predator, leaving behind the red blotches.
Notable patients
- Stephanie Smith, artist who died of SLE complications in 1969 at the age of 22. The anti-Smith (or anti-Sm) antigen was discovered in her and is the basis of a SLE diagnostic test.
- Caroline Dorough-Cochran, sister of Howie D. of the Backstreet Boys, who founded the Dorough Lupus Foundation in her memory.
- Louisa May Alcott, American author.
- Inday Ba (also known as N’Deaye Ba), a Swedish-born actress who died from complications of lupus at age 32.
- Donald Byrne, American chess player who died from complications of lupus in 1976.
- J Dilla (also known as Jay Dee), a hip-hop producer and beatmaker who died of the disease in 2006.
- Hugh Gaitskell, British politician.
- Sophie Howard, British glamour model.
- Michael Jackson, pop superstar, diagnosed with the disease in 1984.
- Charles Kuralt, former anchor of CBS Sunday Morning, died of the disease in 1997.
- Ferdinand Marcos, former Philippine president, who died from complications of lupus in 1989.
- Mary Elizabeth McDonough, American actress.
- Flannery O’Connor, American fiction writer who died of the disease in 1964.
- Elaine Paige, British actress and singer.
- Tim Raines, former major league baseball player, primarily with the Montreal Expos and Chicago White Sox.
- Mercedes Scelba-Shorte, America’s Next Top Model Season Two runner-up and model.
- Seal, singer (discoid lupus of the face, leading to scarring)
- Fred Williamson
- Ray Walston, character actor.
Homeopathy Treatment for Systemic lupus erythematosus
Keywords: homeopathy, homeopathic, treatment, cure, remedy, remedies, medicine
Homeopathy treats the person as a whole. It means that homeopathic treatment focuses on the patient as a person, as well as his pathological condition. The homeopathic medicines are selected after a full individualizing examination and case-analysis, which includes the medical history of the patient, physical and mental constitution, family history, presenting symptoms, underlying pathology, possible causative factors etc. A miasmatic tendency (predisposition/susceptibility) is also often taken into account for the treatment of chronic conditions. A homeopathy doctor tries to treat more than just the presenting symptoms. The focus is usually on what caused the disease condition? Why ‘this patient’ is sick ‘this way’. The disease diagnosis is important but in homeopathy, the cause of disease is not just probed to the level of bacteria and viruses. Other factors like mental, emotional and physical stress that could predispose a person to illness are also looked for. No a days, even modern medicine also considers a large number of diseases as psychosomatic. The correct homeopathy remedy tries to correct this disease predisposition. The focus is not on curing the disease but to cure the person who is sick, to restore the health. If a disease pathology is not very advanced, homeopathy remedies do give a hope for cure but even in incurable cases, the quality of life can be greatly improved with homeopathic medicines.
The homeopathic remedies (medicines) given below indicate the therapeutic affinity but this is not a complete and definite guide to the homeopathy treatment of this condition. The symptoms listed against each homeopathic remedy may not be directly related to this disease because in homeopathy general symptoms and constitutional indications are also taken into account for selecting a remedy. To study any of the following remedies in more detail, please visit the Materia Medica section at Hpathy.
None of these medicines should be taken without professional advice and guidance.
Homeopathy Remedies for Systemic lupus erythematosus :
Agar., alum., alumn., ant-c., apis., arg-n., ars., ars-i., aur-m., bar-c.., bell., calc., carb-ac., carb-v., caust., cic., cist., graph., guare., hep., hydrc., kali-ar., kali-bi., kali-c., kali-chl., kali-s., kreos., lach., lyc., mag-arct., merc-i-r., nat-m., nit-ac., ol-j., paull., phos., phyt., psor., rhus-t., sabin., sep., sil., spong., staph., sulph., thuj., thyr.
References
- ^ “LUPUS FOUNDATION OF AMERICA“. Retrieved on 2007-07-04.
- ^ [https://www.accessmedicine.com/content.aspx?aID=2859070 Harrison’s Internal Medicine, 17th ed. Chapter 313. Systemic Lupus Erythematosus.
- ^ Discoid Lupus Erythematosus
- ^ a b c d “Handout on Health: Systemic Lupus Erythematosus“. The National Institute of Arthritis and Musculoskeletal and Skin Diseases. National Institutes of Health (August 2003). Retrieved on 2007-11-23.
- ^ Lupus: The Great Imitator
- ^ “Lupus: Symptoms – MayoClinic.com“. Retrieved on 2008-07-14.
- ^ a b Joint and Muscle Pain Lupus Foundation of America
- ^ Yu Asanuma, M.D., Ph.D., Annette Oeser, B.S., Ayumi K. Shintani, Ph.D., M.P.H., Elizabeth Turner, M.D., Nancy Olsen, M.D., Sergio Fazio, M.D., Ph.D., MacRae F. Linton, M.D., Paolo Raggi, M.D., and C. Michael Stein, M.D. (2003). “Premature coronary-artery atherosclerosis in systemic lupus erythematosus”. New England Journal of Medicine 349 (Dec. 18): 2407–2414. doi:. PMID 14681506 Abstract (full text requires registration).
- ^ Bevra Hannahs Hahn, M.D. (2003). “Systemic lupus erythematosus and accelerated atherosclerosis“. New England Journal of Medicine 349 (Dec. 18): 2379–2380. doi:. PMID 14681501 Extract (full text requires registration).
- ^ Mary J. Roman, M.D., Beth-Ann Shanker, A.B., Adrienne Davis, A.B., Michael D. Lockshin, M.D., Lisa Sammaritano, M.D., Ronit Simantov, M.D., Mary K. Crow, M.D., Joseph E. Schwartz, Ph.D., Stephen A. Paget, M.D., Richard B. Devereux, M.D., and Jane E. Salmon, M.D. (2003). “Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus”. New England Journal of Medicine 349 (Dec. 18): 2399–2406. doi:. PMID 14681505 Abstract (full text requires registration).
- ^ “General Pathology Images for Immunopathology“. Retrieved on 2007-07-24.
- ^ Anisur Rahman and David A. Isenberg (February 28, 2008). “Review Article: Systemic Lupus Erythematosus“. N Engl J Med 358 (9): 929–939. doi:. PMID 18305268.
- ^ Mary K. Crow (February 28, 2008). “Collaboration, Genetic Associations, and Lupus Erythematosus“. N Engl J Med 358 (9): 956–961. doi:. PMID 18204099.
- ^ Geoffrey Hom, Robert R. Graham, Barmak Modrek, et al. (February 28, 2008). “Association of Systemic Lupus Erythematosus with C8orf13–BLK and ITGAM–ITGAX“. N Engl J Med 358 (9): 900–909. doi:. PMID 18204098.
- ^ University of South Carolina School of Medicine lecture notes, Immunology, Hypersensitivity reactions. General discussion of hypersensitivity, not specific to SLE.
- ^ Gaipl US, Munoz LE, Grossmayer G, et al (2007). “Clearance deficiency and systemic lupus erythematosus (SLE)”. J. Autoimmun. 28 (2-3): 114–21. doi:. PMID 17368845.
- ^ Gaipl, U S; Kuhn, A; Sheriff, A; Munoz, L E; Franz, S; Voll, R E; Kalden, J R; Herrmann, M (2006). “Clearance of apoptotic cells in human SLE“. Current directions in autoimmunity 9: 173–87. PMID : 1639466 Abstract (full text requires registration).
- ^ NIM encyclopedic article on the LE cell test
- ^ Rheumatology.org article on the classification of rheumatic diseases
- ^ Revision of Rheumatology.org’s diagnostic criteria
- ^ a b c d e f g h i j k Edworthy SM, Zatarain E, McShane DJ, Bloch DA (1988). “Analysis of the 1982 ARA lupus criteria data set by recursive partitioning methodology: new insights into the relative merit of individual criteria”. J. Rheumatol. 15 (10): 1493–8. PMID 3060613.
- ^ UpToDate Patient information article on DNA antibodies
- ^ Asherson RA, Cervera R, de Groot PG, et al (2003). “Catastrophic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines”. Lupus 12 (7): 530–4. doi:. PMID 12892393.
- ^ Sangle S, D’Cruz DP, Hughes GR (2005). “Livedo reticularis and pregnancy morbidity in patients negative for antiphospholipid antibodies”. Ann. Rheum. Dis. 64 (1): 147–8. doi:. PMID 15608315.
- ^ Hughes GR, Khamashta MA (2003). “Seronegative antiphospholipid syndrome”. Ann. Rheum. Dis. 62 (12): 1127. doi:. PMID 14644846.
- ^ Hughes GR (1998). “Is it lupus? The St. Thomas’ Hospital “alternative” criteria”. Clin. Exp. Rheumatol. 16 (3): 250–2. PMID 9631744.
- ^ [EARLY STEROIDS MAY PREVENT RELAPSES IN LUPUS, P Jarman (Published in Journal Watch (General) July 18, 1995)